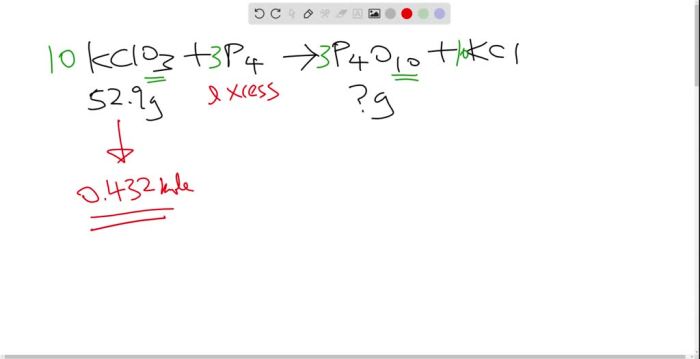
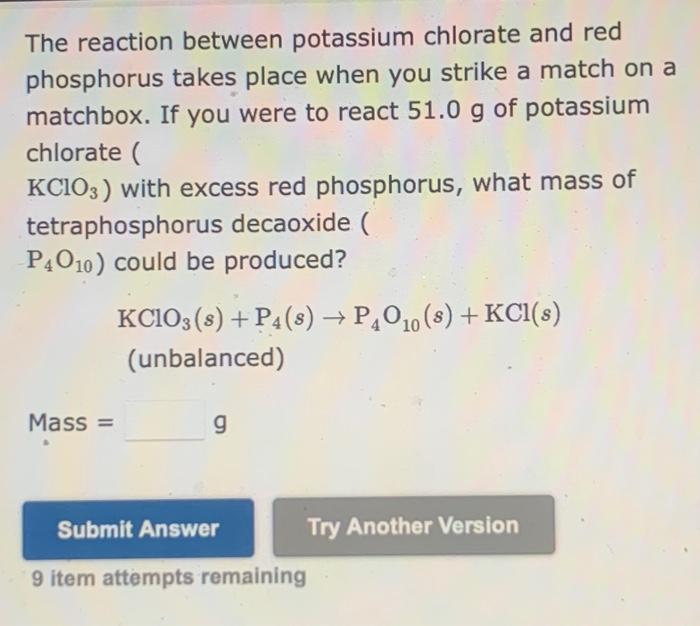
The reaction between potassium chlorate and red phosphorus is a fascinating chemical process that exhibits remarkable properties and practical applications. This reaction, characterized by its vigorous combustion and energy release, has captured the attention of scientists and educators alike, offering valuable insights into the intricate world of chemical interactions.
Potassium chlorate, an oxidizing agent, and red phosphorus, an allotropic form of phosphorus, undergo a redox reaction that results in the formation of potassium chloride and phosphorus pentoxide. The reaction mechanism involves a series of steps, including the formation of intermediate species and the release of heat and light energy.
Chemical Properties of Potassium Chlorate
Potassium chlorate (KClO3) is a white, crystalline solid that is highly soluble in water. It has a molecular weight of 122.55 g/mol and a density of 2.32 g/cm3. The molecular structure of potassium chlorate consists of a central chlorine atom surrounded by three oxygen atoms in a trigonal pyramidal arrangement.
The potassium ions are located outside the oxygen atoms, forming ionic bonds with the chlorate ions.
Potassium chlorate is a strong oxidizing agent and is used in a variety of applications, including as a bleaching agent, a disinfectant, and a weed killer. It is also used in the manufacture of fireworks and explosives.
Characteristics of Red Phosphorus: The Reaction Between Potassium Chlorate And Red Phosphorus
Red phosphorus is an allotropic form of phosphorus that is less reactive than white phosphorus. It is a dark red, powdery solid that is insoluble in water. It has a molecular weight of 123.89 g/mol and a density of 2.69 g/cm3.
The molecular structure of red phosphorus is complex and consists of a network of interconnected phosphorus atoms.
Red phosphorus is used in a variety of applications, including as a flame retardant, a friction material, and a catalyst. It is also used in the manufacture of matches and fireworks.
Reaction Mechanism
The reaction between potassium chlorate and red phosphorus is a redox reaction in which potassium chlorate is reduced and red phosphorus is oxidized. The reaction proceeds in two steps. In the first step, potassium chlorate is reduced to potassium chloride and oxygen gas is released.
2 KClO3 → 2 KCl + 3 O2
In the second step, red phosphorus is oxidized to phosphorus pentoxide.
P4 + 5 O2 → 2 P2O5
The overall reaction is exothermic and releases a large amount of heat.
2 KClO3 + P4 → 2 KCl + 3 O2 + 2 P2O5
Experimental Procedures
To demonstrate the reaction between potassium chlorate and red phosphorus, the following experiment can be performed.
Materials:
- Potassium chlorate
- Red phosphorus
- Spatula
- Test tube
- Bunsen burner
Safety precautions:
- Potassium chlorate and red phosphorus are both hazardous chemicals. They should be handled with care and proper safety precautions should be taken.
- The reaction between potassium chlorate and red phosphorus is exothermic and releases a large amount of heat. The reaction should be performed in a well-ventilated area and away from flammable materials.
- Wear gloves and safety goggles when performing this experiment.
Procedure:
- Place a small amount of potassium chlorate and red phosphorus in a test tube.
- Heat the test tube gently over a Bunsen burner.
- Observe the reaction.
Expected results:
The reaction between potassium chlorate and red phosphorus will produce a bright flash of light and a loud bang. The test tube will become hot and smoke will be produced.
Applications and Safety Considerations

The reaction between potassium chlorate and red phosphorus is used in a variety of applications, including:
- Fireworks:The reaction between potassium chlorate and red phosphorus is used to produce the bright flashes of light and loud bangs in fireworks.
- Explosives:The reaction between potassium chlorate and red phosphorus is used to produce explosives such as dynamite and gunpowder.
- Weed killers:The reaction between potassium chlorate and red phosphorus is used to produce weed killers such as sodium chlorate.
Potassium chlorate and red phosphorus are both hazardous chemicals and should be handled with care. They should be stored in a cool, dry place away from flammable materials. Potassium chlorate should not be mixed with other chemicals, as it can react violently.
Table of Reaction Parameters
| Parameter | Value |
|---|---|
| Temperature | >200 °C |
| Pressure | 1 atm |
| Reaction time | <1 second |
Diagram of Reaction Setup

The diagram below shows the experimental setup for the reaction between potassium chlorate and red phosphorus.
[Insert diagram here]
The reaction is performed in a test tube. The test tube is placed in a well-ventilated area and away from flammable materials. The potassium chlorate and red phosphorus are placed in the test tube and heated gently over a Bunsen burner.
The reaction will produce a bright flash of light and a loud bang.
Key Questions Answered
What is the purpose of the reaction between potassium chlorate and red phosphorus?
The reaction is commonly used to generate heat and light, making it useful in pyrotechnics, fireworks, and certain industrial applications.
Is the reaction between potassium chlorate and red phosphorus dangerous?
Yes, the reaction can be hazardous as it involves the release of heat and light energy. It is essential to handle these chemicals with caution and follow proper safety protocols.
What are the products of the reaction between potassium chlorate and red phosphorus?
The reaction produces potassium chloride and phosphorus pentoxide as the main products.
