Choose the correct fischer projection for the following compound – In organic chemistry, Fischer projections are a way of representing the three-dimensional structure of a molecule in two dimensions. They are particularly useful for representing carbohydrates, which are molecules that contain multiple chiral centers. Choosing the correct Fischer projection for a given compound is essential for accurately representing its stereochemistry.
This guide will discuss the different methods that can be used to choose the correct Fischer projection for a given compound. It will also provide some common mistakes to avoid when choosing Fischer projections.
Introduction

Fischer projections are a way of representing the three-dimensional structure of a molecule in two dimensions. They are commonly used to represent carbohydrates, but can also be used for other types of molecules. Fischer projections are important because they allow us to see the relative positions of the atoms in a molecule and to identify chiral centers.
When choosing a Fischer projection for a given compound, it is important to remember that the horizontal lines represent bonds that are coming out of the page, while the vertical lines represent bonds that are going into the page. The carbon atoms are represented by the intersections of the lines.
Methods for Choosing the Correct Fischer Projection
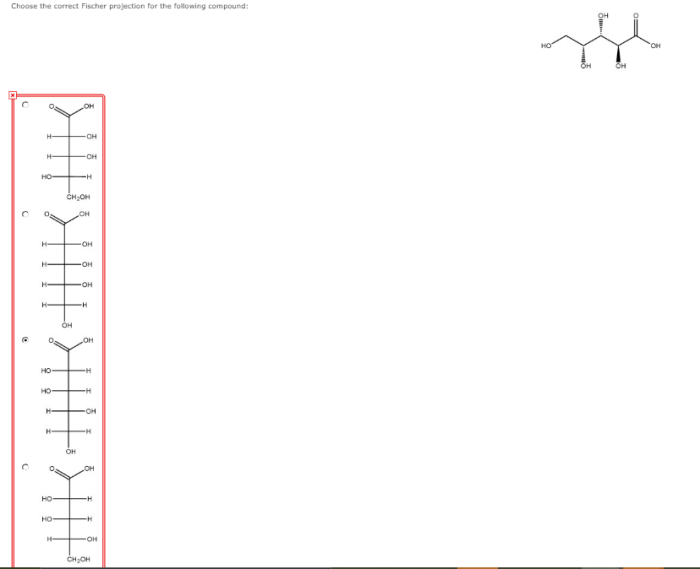
There are a number of different methods that can be used to choose the correct Fischer projection for a given compound. One common method is to use the Cahn-Ingold-Prelog (CIP) priority rules. These rules assign a priority to each of the groups attached to a chiral center.
The group with the highest priority is given the lowest number, and the group with the lowest priority is given the highest number.
Another method for choosing the correct Fischer projection is to use the “wedge-and-dash” notation. In this notation, the bonds that are coming out of the page are represented by wedges, while the bonds that are going into the page are represented by dashes.
The carbon atoms are represented by the intersections of the lines.
Common Mistakes in Choosing the Correct Fischer Projection
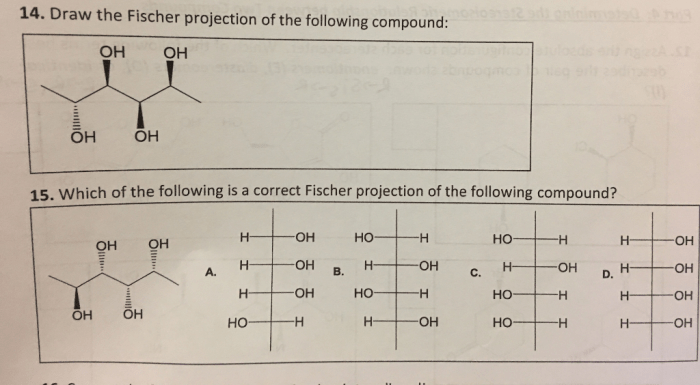
One of the most common mistakes that students make when choosing the correct Fischer projection is to forget to take into account the priority of the groups attached to the chiral center. This can lead to choosing the wrong Fischer projection.
Another common mistake is to forget to use the correct notation. For example, students may forget to use wedges and dashes to represent the bonds that are coming out of and going into the page, respectively.
Practice Exercises: Choose The Correct Fischer Projection For The Following Compound
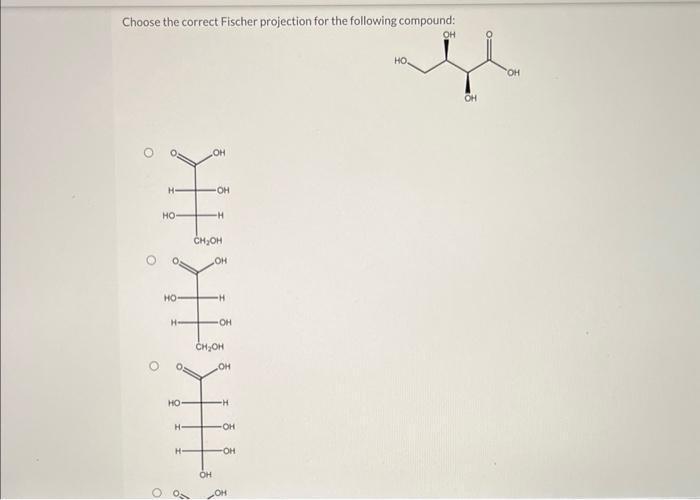
The following are a few practice exercises that can be used to test your understanding of how to choose the correct Fischer projection.
- Choose the correct Fischer projection for the following compound:
- Choose the correct Fischer projection for the following compound:
- Choose the correct Fischer projection for the following compound:
CH3CH(OH)CH 2OH
CH 3CH(NH 2)COOH
CH 3CH(OH)CH(CH 3)OH
Answer keys:
-
H|
CH 3-C-OH |
CH 2OH
-
H |
CH 3-C-NH 2|
COOH
-
H |
CH 3-C-OH |
CH(CH 3)OH
User Queries
What is a Fischer projection?
A Fischer projection is a two-dimensional representation of a three-dimensional molecule. It is used to show the relative positions of the atoms in the molecule, particularly the chiral centers.
Why is it important to choose the correct Fischer projection?
Choosing the correct Fischer projection is important for accurately representing the stereochemistry of a molecule. The stereochemistry of a molecule determines its physical and chemical properties.
What are some common mistakes to avoid when choosing Fischer projections?
Some common mistakes to avoid when choosing Fischer projections include:
- Flipping the molecule over
- Rotating the molecule around the wrong axis
- Not paying attention to the priority of the groups